Abstract
Background:
The interfering effects of DOACs on the screening coagulation tests, such as prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen assay, have been shown mainly by in vitro spiking experiments. However, the effects of DOACs on coagulation tests in real-world samples from anticoagulated patients are unknown because of the difficulty in selectively eliminating DOAC from blood samples already containing DOACs.
Method:
Citrated blood samples were drawn from patients on anticoagulation therapy (rivaroxaban and edoxaban). In addition, blood samples from patients not on anticoagulation were collected. PT INR and APTT were measured from those samples by coagulometers from two manufacturers (Roche t711, Swiss and ACL-TOP, USA). We also measured DOAC levels from the same samples by anti-FXa activity (Hyphen Biomed, France). Then, we compared the test results in relation to the DOAC levels.
Results:
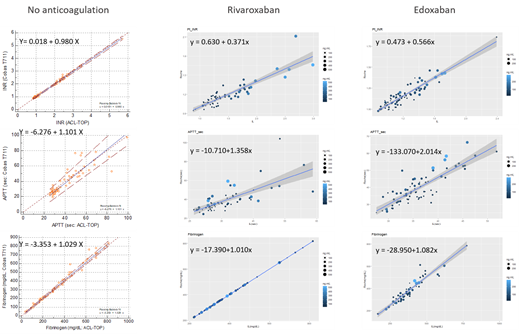
The PT INR, APTT, and fibrinogen assay results from non-anticoagulated patients measured by the two coagulometers were comparable (PT INR: y = -3.353 + 1.029 x; APTT: y = -6.276 + 1.101x; fibrinogen: y = -3.353 + 1.029 x; Passing Bablok). We included blood samples from 61 patients on rivaroxaban and 75 patients on edoxaban. From the rivaroxaban samples we observed the regression line change for PT INR (y = 0.6303 + 0.3712x) and for APTT (y = -10.71+1.358x). The comparability of fibrinogen assay was not affected significantly (y = -17.39+1.01x). From the edoxaban samples we also observed the similar change of the regression line (PT INR: y = 0.4728 + 0.5661x; APTT: y = -133.07+2.014x). Fibrinogen levels were comparable (y = -28.95+1.082x).
Conclusion:
The susceptibility of screening coagulation tests to the interfering effects of in vivo DOAC is dependent on the reagents and coagulometers.
No relevant conflicts of interest to declare.